The Importance of Clinical Trial Data Security
The Importance of Clinical Trial Data Security
In a world that is increasingly digitized, the security of data has become one of the foremost concerns for every business, industry, and organization. One sector that is particularly sensitive to the importance of data security is healthcare, more specifically, clinical trials. The delicate, confidential, and highly personal nature of the data collected during clinical trials makes their security vital, not only for the integrity and success of the trial itself but also to uphold the trust of the participants.
As a part of healthcare, clinical trials have the same obligation to uphold the privacy and security of their participants as their sector counterparts. Their role is unique, involving the collection and analysis of large amounts of data from diverse patient populations to investigate the efficacy and safety of new drugs or treatments. The sensitive information involved extends beyond personal health history to include genetic information, treatment records, lab results, and sometimes even lifestyle information. This conglomerate of sensitive data, therefore, becomes a notorious target for cyber-attacks and unauthorized access. Ensuring the security of clinical trial data is hence not only ethically right but also a regulatory and legal requirement.
In an era where a vast majority of this sensitive data is stored, processed, and transferred digitally, the risks of data breaches and cyber-attacks have increased dramatically. It is not uncommon to hear about sophisticated cyber threats compromising the data security of healthcare organizations. This presents a new set of challenges for organizations conducting clinical trials, who are now required to ensure robust security protocols are in place from data collection to storage, processing, dissemination, and eventual destruction. Advancements in new technology may bring convenience and efficiency, but they also introduce substantial risks.
Clinical trial data is also a key driver in many business decisions. It influences regulatory approvals, investment decisions, and marketing strategies in the pharmaceutical industry. A breach in clinical trial data can therefore have wide-reaching implications, compromising the validity of the results, the reputation of the involved parties, and even slowing the speed at which new treatments make it to market. Organizations face the risk not only of regulatory penalties but also the loss of trust that can be devastating to their brand image and future business prospects.
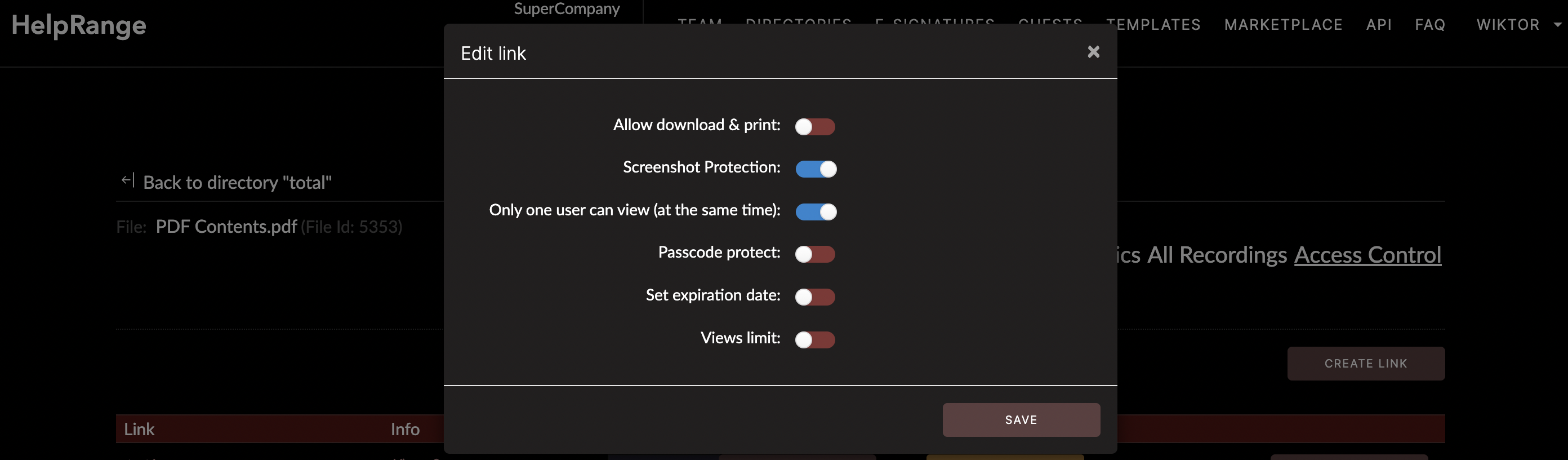
So how can an organization ensure clinical trial data security? The answer lies in implementing a comprehensive and robust data management strategy that blends cutting-edge technology with stringent security measures. One key aspect is through the protection of documents, especially PDFs, which play a substantial role in clinical trials. From consent forms to study protocols, data reports, and regulation documentation, the ability to restrict access, control use, and track engagement of these critical documents is paramount. A wide range of online tools available can offer PDF protection, usage analytics and tooling. An example includes platforms like HelpRange, which provides versatile solutions to protect, control and monitor PDF files in real-time online.
Apart from document protection, other important measures should include strong password systems, two-factor authentication, encryption of data at rest and in transit, regular software updates, training and awareness programs for staff, and a comprehensive incident response plan for rapid containment and recovery from any potential breaches.
In conclusion, clinical trial data security is not a negotiable aspect of any clinical trial. It is an integral part of the planning, conduct, and even post-trial operations, without which the entire work may not only risk regulatory penalties but also reputational loss, financial consequences, and a breach of trust with participants. By employing robust data security measures and maintaining active vigilance, organizations can reduce the risk of data breaches, uphold trust, and ensure the successful execution of clinical trials in their quest to improve healthcare.
Ultimately, enhancing data security is not just about protecting personal information or meeting legal requirements; it's about fostering an environment of trust where participants can feel confident that their information is kept private and secure. It's about demonstrating integrity and commitment to transparency, which in turn can have a positive impact on patient participation rates and the overall success of clinical trials. It's about ensuring the viability and trustworthiness of the clinical trial enterprise as a whole.
In a world where data is the new currency, clinical trial data security needs as much attention as the trials themselves. By interweaving robust security measures throughout every clinical trial phase, organizations can ensure the privacy, safety, and trust of every participant – now, and in the future.
Check out HelpRange
HelpRange is "Next-Gen Documents Protection & Analytics Platform". HelpRange represents the cutting-edge platform for document access controls and in-depth analytics, ensuring superior management and usage insights for your documents.
